Contrast-Enhanced Ultrasound Expands, Seeks to Clear Regulatory and Payment Hurdles
By offering benefits of cost-effectiveness, convenience, and safety, contrast-enhanced ultrasound (CEUS) has found an increasing number of applications, and its advocates are pushing for removing barriers to its use in a growing number of areas in medicine.
The use of ultrasound contrast agents (UCAs) is key to the technology, which are liquid suspensions of gas-filled microbubbles that are injected intravenously during the ultrasound exam to produce high-quality, reliable diagnostic images. CEUS is exceedingly safe, as patients are not exposed to ionizing radiation and UCAs do not contain dye. CEUS may also cut costs of diagnostic imaging by improving interpretability and reducing the need for additional studies. Furthermore, unlike CT or MRI, no imaging suites are necessary since ultrasound can be conducted at bedside.
|
|
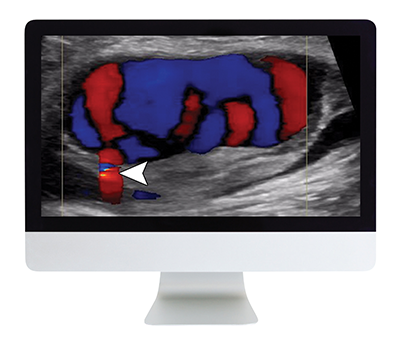 |
| Focus on honing your skill in ultrasound with this course which combines 13 traditional case reviews with 4 didactic lectures for deeper insights. |
Among recent developments in the field, research has found that a CEUS scan of the kidney can be more accurate than CT and MRI in predicting whether suspicious masses are cancerous, potentially reducing the need for additional biopsies and surgeries. One study followed 721 patients with approximately 1,000 kidney masses for up to 10 years. Following CEUS, 367 patients were spared biopsy, surgery, or close follow-up. Five patients previously thought to have benign lesions were found instead to have cancerous tumors.
In a subgroup of patients initially believed to have a high probability of malignancy, the use of CEUS showed 78% of the tumors were not malignant. In another subgroup of patients thought to have a 100% chance of malignancy, 38.7% of the kidney masses were found to be nonmalignant.
Additional research released in 2018 described how patients with hepatocellular carcinoma, the third leading cause of cancer deaths worldwide, were found to benefit from a combination of CEUS and radiation therapy. The study compared one group of patients with liver cancer who were treated with radiation therapy alone with another group who received radiation therapy combined with CEUS. It was found that, among patients receiving a combination of CEUS and radiation therapy, the number of vessels carrying blood to the tumor were reduced along with a 25– 60% increased response to the radiation treatment. The findings appear to be consistent with previous work using the microbubbles to enhance absorption of chemotherapy drugs.
“These are exciting preliminary findings because hepatocellular carcinoma is the most common form of liver cancer, and microbubbles appear to sensitize the tumor to the radiation therapy,” said John Eisenbrey, a researcher at Thomas Jefferson University, in a news release.
Along with new developments in the use of CEUS, last year the International Contrast Ultrasound Society (ICUS) hailed changes in Medicare physician payment rules that will support the use of CEUS for noncardiac abdominal imaging. The changes in federal policy, which took effect January 1, 2019, include a new “Category I” Current Procedural Terminology (CPT) code, which ICUS said would improve patient access to CEUS.
ICUS advocates for the safe and appropriate use of CEUS to improve patient care. In announcing the changes, ICUS thanked ARRS and a number of other professional societies and medical organizations for their help in bringing about the new CPT code.
“The upgraded professional fees and new Category I CPT code represent a potential sea change for the ultrasound community,” according to Dr. Ed Grant, treasurer of ICUS and professor and chairman, Department of Radiology, Keck School of Medicine, University of Southern California.
|
|
 |
| Review the current status of imaging and protocols for a variety of suspected or known disorders in the emergency setting. |
Additionally in 2018, ICUS called for the U.S. Food and Drug Administration (FDA) to remove boxed warnings from UCAs, emphasizing that studies show UCAs to be extremely safe. ICUS stated in its petition that UCAs present no known risk of kidney or liver damage and are expelled from the body within minutes, adding that the boxed warnings are appropriate only as an indicator of the very highest risk level associated with FDA-approved products.
In a news release, ICUS co-president Stephanie Wilson, professor of radiology at the University of Calgary, stated that the United States is currently behind the rest of the world when it comes to using CEUS. Though UCAs are approved by the FDA for cardiac and liver imaging in adults and children in Europe, Canada, Asia, and Brazil, UCAs are being used to pinpoint cancers elsewhere in the body, as well as to monitor chronic gastrointestinal diseases, detect vascular disease, and diagnose other conditions. ICUS noted in its petition that the FDA has steadily responded to evidence of the safety and efficacy of UCAs by downgrading package insert contraindications three times since 2007 and by removing a 30-minute monitoring requirement for patients with pulmonary hypertension or unstable cardiopulmonary conditions.
The cost savings from using CEUS can be significant. A study released last year showed that Los Angeles County Hospital saved $117,000 annually by using CEUS in an initial scan to immediately detect liver and kidney lesions without waiting for further CT or MRI procedures. Conducting an initial CEUS scan avoided further CT or MRI procedures in two-thirds of patients studied.
In the study, the hospital’s cost of a CEUS scan was estimated at $309, compared with the cost of CT ($647.31) or MRI ($1,116.94), based on Medicare costs. In addition, the study reported that the time to diagnosis using CEUS was 5.2 days, compared with 52 days for CT and 123.5 days for MRI.